Identification of T/B-like cell-specific transcription factors in Lamprey ~ evolution of adaptive immunity and related-transcription factors ~

Max Plank Institute of
Immunobiology and Epigenetics
(ドイツ)
Ryo Morimoto
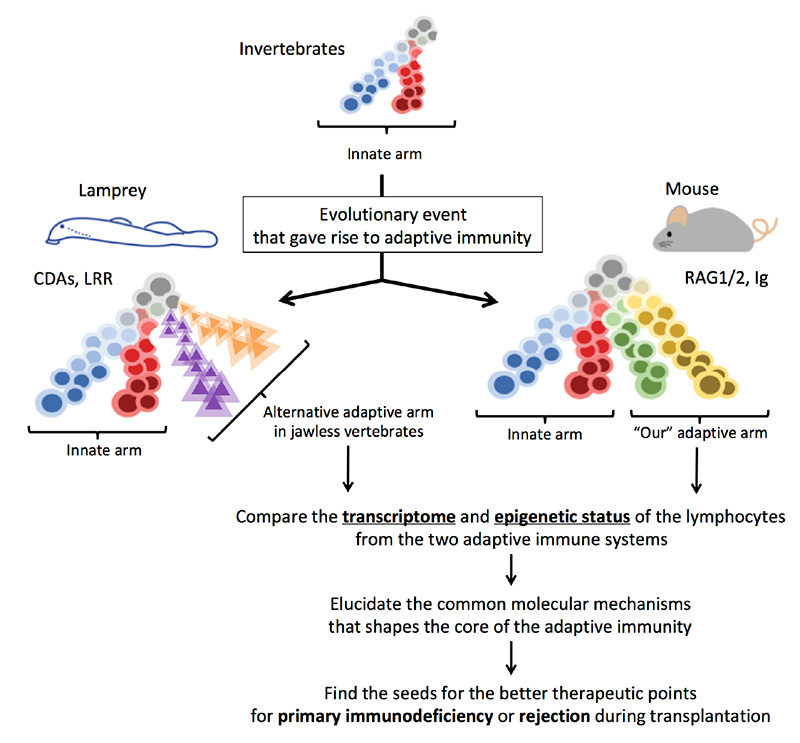
Lymphocyte-based adaptive immunity is observed only in vertebrate species. The main purpose of the project is to explore the core molecular mechanisms supporting the development of adaptive immune system by comparing two distinct systems to recognize diverse antigens. Jawed vertebrate (from shark to human) has adaptive immunity that relies on Rag1/Rag2-mediated assembly of antigen receptor genes (T cell receptor and immunoglobulin) in T and B lymphocytes. In contrast, jawless vertebrates (lamprey and hagfish) have alternative antigen recognition system consisting of leucine-rich repeat arrays encoded by somatically assembled variable lymphoid receptor (VLR) genes. Despite of the different molecular entities, they still show T-like and B-like cells that play roles in the self-defence in a similar manner to ours. By focusing on these differences and the common design principle, we will investigate the evolutionary history of the molecular mechanisms that gave rise to the noble antigen recognition system. Here, modern technologies will be applied to a non-conventional model animal, lamprey, leading us to better understanding of the molecular network of the evolution and the development of lymphocytes in vertebrates. The results expected in the project will also provide new perspectives on the primary immunodeficiency and pathophysiology of rejection process in allogenic transplantation.