Exploring the mechanism of sodium-induced inflammation using CRISPR screening and ribosome footprinting.

Max-Delbrück-Centrum
für Molekulare Medizin
(ドイツ)
Dominik N. Müller
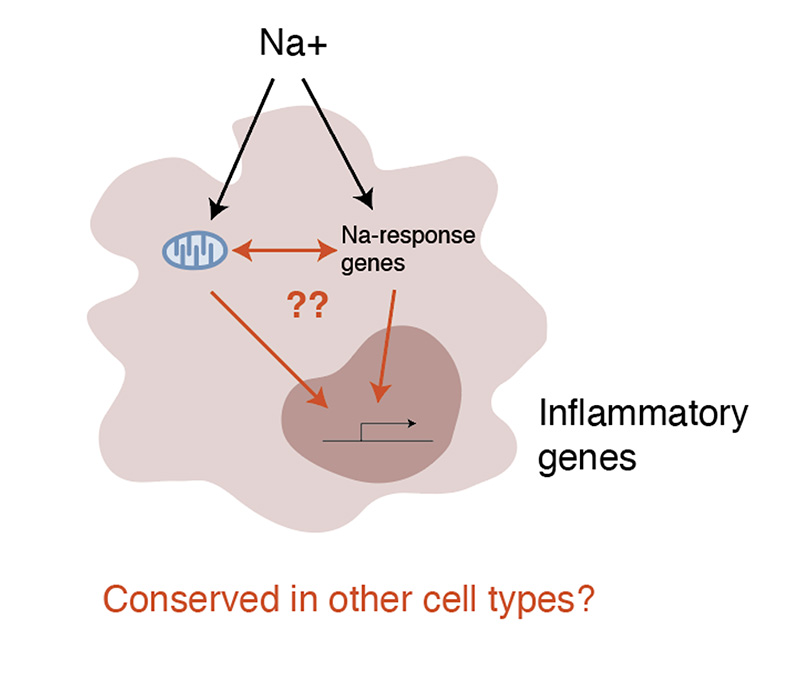
Excessive salt intake due to the widespread Western diet has become a major health problem. The role of sodium (Na+) as a second messenger is not fully understood. In the development of salt-related diseases, innate and adaptive immune cells trigger increased inflammation in the presence of high Na+ levels. Our goal is to identify the molecular mechanism to provide a solid foundation for further understanding of the mechanisms by which cells sense and cope with Na+ and to develop novel interventions/treatments for salt-related diseases based on their molecular pathogenesis. Previously, in CRISPR screening of a macrophage cell line, we found genes associated with the sodium response pathway. In parallel, our laboratory discovered that the mitochondrial electron transport chain (ETC) is disrupted by Na+ and that this disruption enhances inflammation in macrophages and T cells. Since then, we have focused on studying the relationship between putative sodium-response genes and ETC inhibitor/Na+. In this project, we will investigate how ETC disturbance causes inflammation and whether this mechanism is also found in other immune cells such as T cells.