RESEARCH
Study on the mechanisms of cell fate decision in hematopoiesis and development of a cell therapy using T cells regenerated from iPS/ES cells
The major aim of our laboratory is to elucidate the molecular mechanisms that regulate cell fate decisions in the process of lineage restriction from multipotent hematopoietic stem cells to unipotent progenitors. Among various events occurring during hematopoiesis, we are mainly focusing on the process from hematopoietic stem cells to T cells.
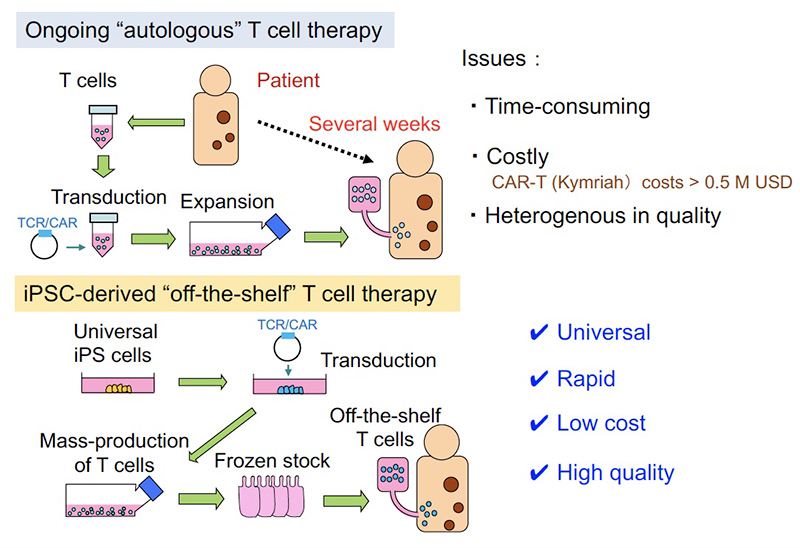
As another project, we have been developing an approach aiming to apply our culture method in clinical settings. In currently ongoing adoptive T cell therapies, T cells collected from patient are transduced with CAR or TCR genes and given back to the patient after ex-vivo activation and expansion. While such strategies using autologous T cells have been shown to be effective in some types of cancer, some issues remain to be solved: i) time-consuming and ii) costly, and iii) difficult to guarantee the quality because the products depend on patient-derived T cells. To solve these problems, we thought of a method in which cytotoxic T lymphocytes (CTLs) can be mass-produced by the iPS cell technology. Our initial concept is the following; iPSCs produced from T cells (T-iPSCs) inherit rearranged T cell receptor (TCR) genes, and thus all regenerated T cells from T-iPSCs express the same TCR. Based on this idea, we succeeded in regenerating tumor antigen-specific CD8 T cells (Cell Stem Cell, 2013). We then developed a culture method by which potent CTLs expressing CD8αβ can be generated (Cancer Research, 2016). To apply this approach in allogeneic setting, we next developed a method in which non-T cell derived iPSCs are transduced with exogenous TCR genes (TCR-iPSC method) (Mol Ther Methods Clin Dev, 2020). We are now preparing for clinical trial to be realized in Kyoto University Hospital, in which acute myeloid leukemia patients will be treated by the regenerated CTLs. We are also developing an immune cell therapy against viral infections, such as COVID-19 or cytomegalovirus infection reactivated after bone marrow transplantation.
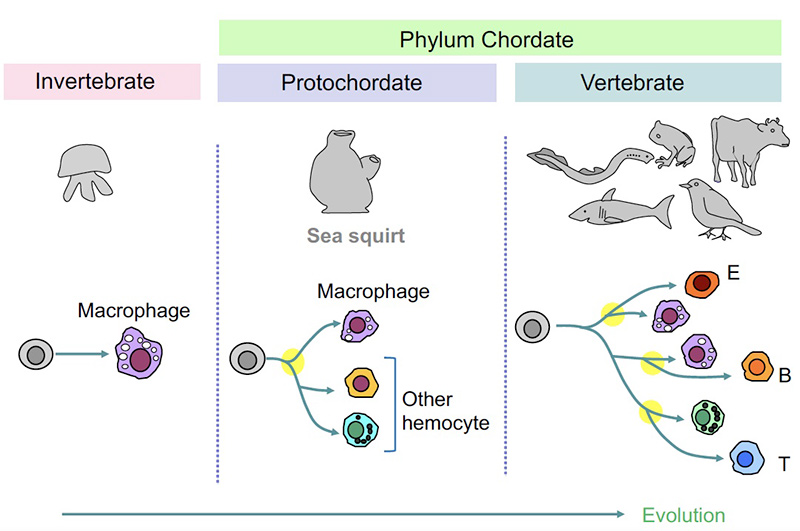
Blood cells have diversified from phagocytes during evolution
Very primitive invertebrates solely have phagocytes but not other types of blood cells. Sea squirt belongs to Chordate family, which we vertebrates also belong, representing our ancestor. In sea squirt or in more advanced creatures, various types of blood cells can be seen in the body. We think that such blood cells have independently evolved from phagocytic cells during the evolutionary history.
Issues in the ongoing adoptive T cell therapy and solution by “universal” and “off-the shelf” T cell medicine
In currently ongoing adoptive T cell therapies, T cells collected from patient are transduced with CAR or TCR genes and given back to the patient after ex-vivo activation and expansion. While such strategies using autologous T cells have been shown to be effective in some types of cancer, some issues remain to be solved: i) time-consuming and ii) costly, and iii) difficult to guarantee the quality because the products depend on patient-derived T cells. To solve these problems, we are developing a method in which cytotoxic T lymphocytes (CTLs) can be mass-produced from the iPS/ES cells.