研究分野
Exploring the virus replication mechanism
Our laboratory has been investigating the replication mechanisms of influenza virus, Ebola viruses, and SARS-CoV-2. One of the most unique features of our research is imaging and structural analysis, by combining conventional virological techniques with various microscopic methods such as cryo-electron microscopy and high-speed atomic force microscopy. We also investigate virus replication and host responses against virus infection using human ES- and iPS-derived organoids, which mimic virus infection in the human body. Our unique approaches will provide important insights into virus replication mechanisms and advance our understanding of the molecular and structural basis of the replication mechanism.
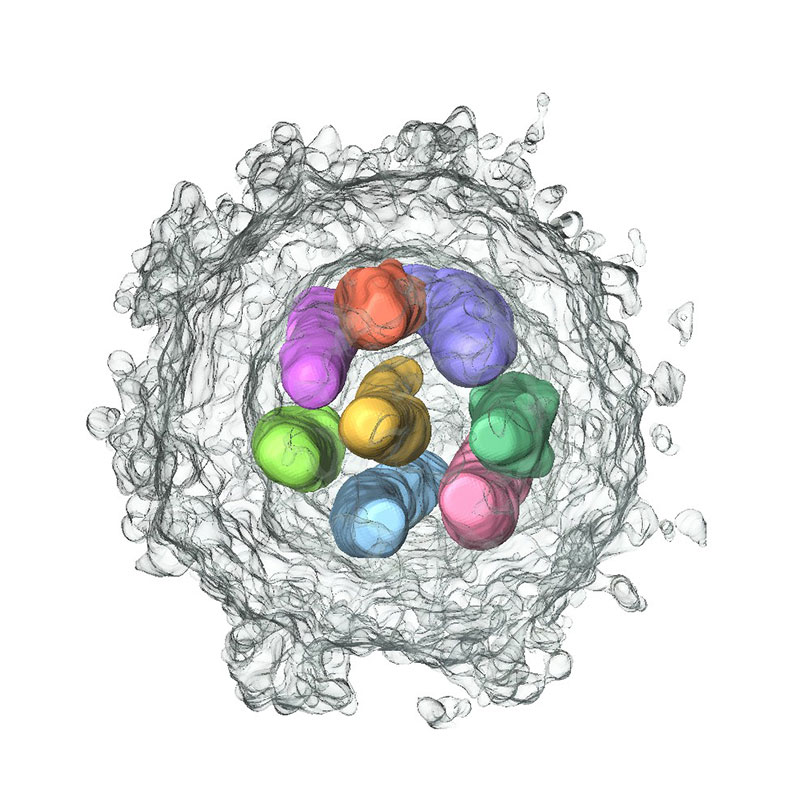
Genome packaging mechanism of influenza virus
The influenza virus genome is segmented into 8 pieces. However, it has long been a mystery how these 8 genomic segments are incorporated into progeny virions. By combining reverse genetics and various electron microscopy techniques, we demonstrated that the 8 different genome segments arranged in a specific pattern are selectively incorporated into each virion. We are currently working to elucidate the molecular mechanisms behind this process. (Figure)Influenza virus particle 3D-reconstructed using electron tomography.
[References:Noda et al. Nature 2006; Muramoto et al. J Virol 2006; Noda et al. Nat Commun 2012; Sugita et al. J Virol 2013; Noda et al. Nat Commun 2018; Miyamoto et al. J Virol 2022]
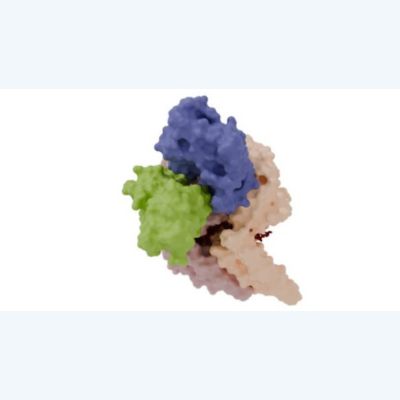
Mechanisms of Ebola virus nucleocapsid formation and its functional regulation
Ebola virus nucleocapsid is a large helical complex composed of genome RNA and proteins NP, VP24, VP35, VP30, and L, which plays various roles such as genome RNA transcription, replication, and transport to progeny viral particles. However, its detailed structure remains unresolved. Using cryo-electron microscopy, we determined the high-resolution structure of the helical NP-RNA complex, which becomes the core of the nucleocapsid. Furthermore, we also determined the high-resolution structure of the NP-RNA complex bound to VP24 molecules, elucidating the transcription and replication regulation mechanism of the nucleocapsid by VP24. (Figure) Cryo-EM structure of the Marburg virus NP-RNA complex.
[References: Noda et al. PLoS Pathog 2006; Noda et al. J Gen Virol 2010; Sugita et al. Nature 2018; Fujita-Fujiharu et al. Nat Commun 2022; Hu et al. PNAS Nexus 2023]

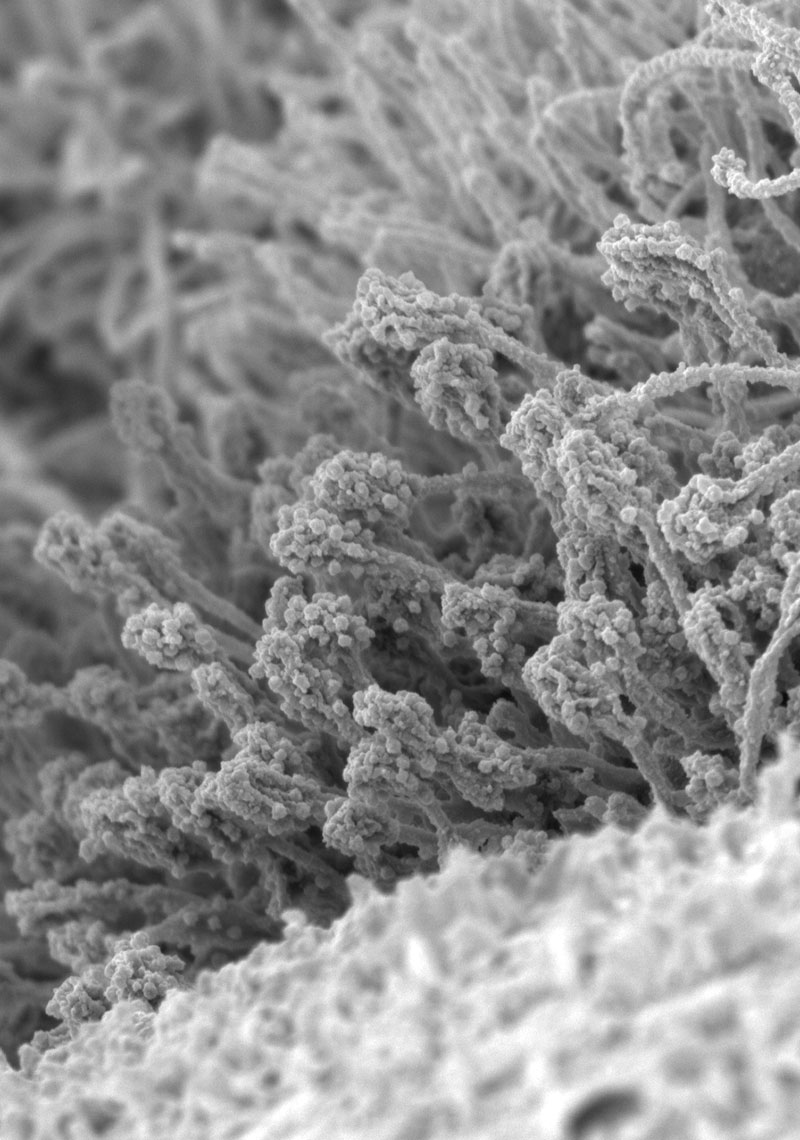
Development of human respiratory organoids for a novel virus infection model
In conventional virus research, it is common to use cultured cell lines derived from various animal species and organs that are not the natural targets of the virus, which often fail to accurately reflect the infection and replication mechanisms or host immune responses in our body. To address this concern, we have been working on developing human respiratory epithelial cells from ES cells and iPS cells for virus research and advancing the analysis of virus replication mechanisms and host immune responses in human respiratory tract. (Figure) Scanning electron microscope image of human nasal epithelial organoids derived from ES cells.
[Reference: Muramoto et al. Lancet Microbe 2023]