RESEARCH
Development and implementation of basic research for the establishment and utilization of human ES cell lines for clinical use
Regenerative medicine using cells and tissues derived from human ES cells and human iPS cells has attracted worldwide attention. It is currently in the clinical trial stage, where safety and efficacy are confirmed. Our laboratory is engaged in establishing human ES cell lines for clinical use, stock production, and distribution to (clinical) research facilities. At the same time, we are developing the necessary fundamental technologies for this purpose.
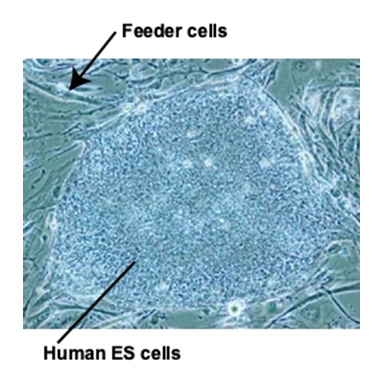
Phase contrast photograph of the first human ES cells established in Japan
As shown in the photo, human ES cells were cultured with support cells called feeder cells in culture media containing animal components, which was unsuitable for clinical use. The development of culture substrates and culture media was essential for the realization of regenerative medicine using human ES cells and human iPS cells.
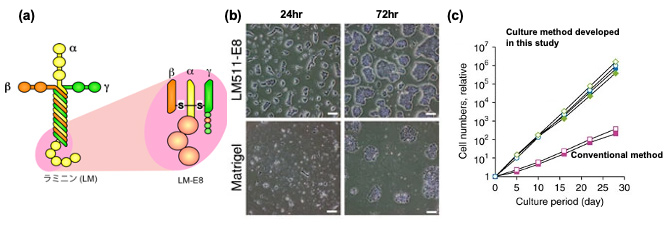
Development of an efficient human pluripotent stem cell culture method using the LM-E8 Fragment
Matrigel was conventionally used as a culture substrate for human ES cells and human iPS cells, but it was not suitable for practical use in regenerative medicine. In collaboration with Prof. Sekiguchi of Osaka University, we found that Laminin-511 is appropriate and that its E8 fragment (a) is the minimal constitutive site. Using this LM-E8 fragment, we found that cells grow, survive, and increase more efficiently than Matrigel (b,c). This EM-E8 fragment has been commercialized as iMatrix and is used worldwide.
Adapted in part from Nature Communication (DOI: 10.1038/ncomms2231)
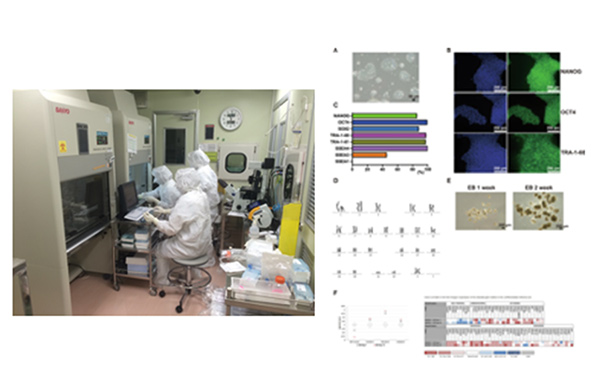
Photographs of work in the cell processing facility at the institute (Left) and some of the data from the clinical human ES cell line (KthES11) established in this facility (Right).
Adapted in part from Stem Cell Research (DOI: 10.1016/j.scr.2020.102020)
TOPICS

2025.10.8
Identification of a compound that lowers the cost of human ES/iPS cell culture while enhancing iPS cell generation
2022.11.9
Successful establishment and development of efficient clinical human ES cell lines and banking of cell lines
2021.6.16
Establishment of a clinical-grade human embryonic stem cell line KthES11



