RESEARCH
Invent novel biotechnology leveraging micro/nanoscale mechanics
Established in April 2023, the Nano Bioengineering Lab delves into the intricate organization of multicellular systems. Our research endeavors focus on elucidating the cross-scale system from single cells to complex organisms and understanding the mechanisms underlying diseases that arise from organizational disruptions. We pioneer innovative omics methodologies, leveraging advancements in micro/nanofluidics and electrokinetics, to unravel these fundamental questions. Our interdisciplinary approach unites experts from diverse fields including basic biology, cancer biology, biophysics, and mechanical engineering. As a recent achievement, we developed ELASTomics (electroporation-based lipid-bilayer assay for cell surface tension and transcriptomics), which measures cell surface tension and gene expression with single-cell resolution. Additionally, we created an optical-indexing method that integrates microscopic images and gene expression by combining fluorescent color codes and DNA barcodes. At the heart of our work lies the collaborative environment of LiMe, serving as a dynamic hub for interdisciplinary exchange. Together, we strive to push the boundaries of knowledge and contribute to breakthroughs in biomedical science and engineering.
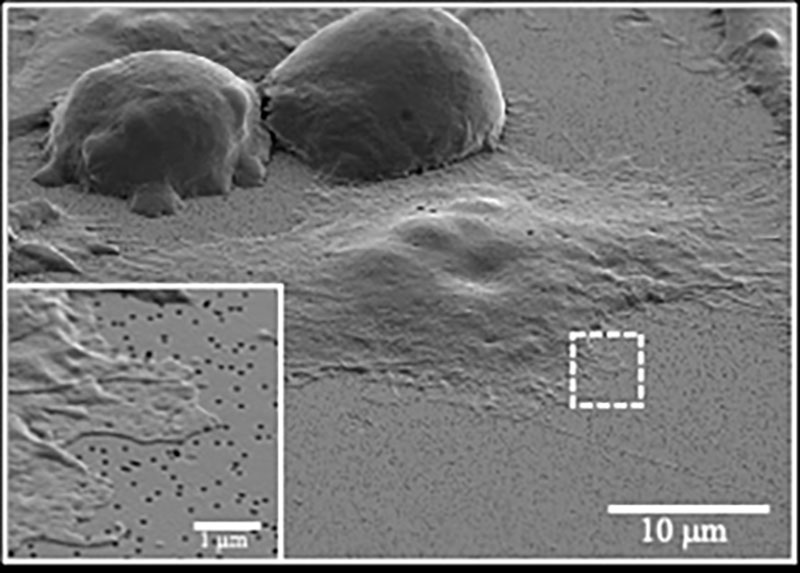
Measurement of cell surface tension and gene expression at single-cell resolution (ELASTomics)
The mechanical properties of cells are known to be associated with physiological states in various biological contexts, such as cell differentiation, cancer, and aging. To elucidate the intricate molecular mechanisms governing the mechanical properties of cells, we have developed a novel method capable of integrating cell mechanics profiling with unbiased transcriptomics for thousands of single cells (Shiomi et al., Nat Commun., 2024).
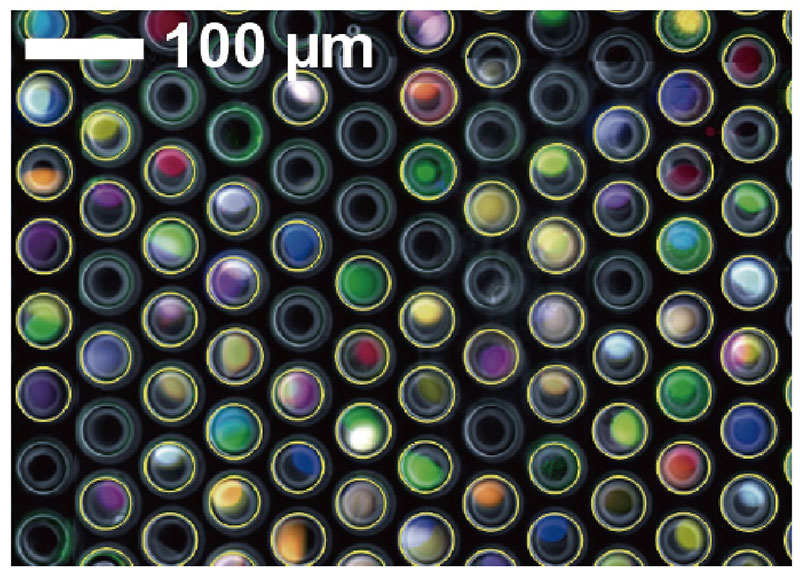
Opto-combinatorial indexing that integrates microscopic images and gene expression of single cells
Genetically identical cells can show diverse responses to drugs, leading to different cell fates, such as drug resistance in cancer. To study the molecular cascade of how the variation in gene expression gives rise to different phenotypic responses, we introduced a method combining optical indices from cells and hydrogel beads with single-cell RNA sequencing, linking cellular drug responses to the gene expression variations (Tsuchida et al., LabChip, 2024).