| Jan 31, 2023 Murine breast cancers disorganize the liver transcriptome in a zonated manner |
Alexis Vandenbon1,2,#,*, Rin Mizuno3,4,#, Riyo Konishi3,#, Masaya Onishi5,#, Kyoko Masuda6, Yuka Kobayashi6, Hiroshi Kawamoto6, Ayako Suzuki5, Chenfeng He3,7, Yuki Nakamura3,7, Kosuke Kawaguchi7, Masakazu Toi7, Masahito Shimizu8, Yasuhito Tanaka9, Yutaka Suzuki5,*, Shinpei Kawaoka3,10,*
1 Laboratory of Tissue Homeostasis, Institute for Life and Medical Sciences, Kyoto University.
2 Institute for Liberal Arts and Sciences, Kyoto University.
3 Inter-Organ Communication Research Team, Institute for Life and Medical
Sciences, Kyoto University
4 Department of Gynecology and Obstetrics, Kyoto University Graduate School of Medicine
5 Graduate School of Frontier Science, The University of Tokyo
6 Laboratory of Immunology, Institute for Life and Medical Sciences, Kyoto University
7 Department of Breast Surgery, Kyoto University Graduate School of Medicine
8 Department of Gastroenterology/Internal Medicine, Gifu University Graduate School of Medicine
9 Department of Gastroenterology and Hepatology, Faculty of Life Sciences, Kumamoto University
10 Department of Integrative Bioanalytics, Institute of Development, Aging and Cancer
(IDAC), Tohoku University
#These authors equally contributed to this work.
*Corresponding Authors
Murine breast cancers disorganize the liver transcriptome in a zonated manner
Communications Biology, 6, 97 (2023) doi.org/10.1038/s42003-023-04479-w
Abstract
The spatially organized gene expression program within the liver specifies hepatocyte functions according to their relative distances to the bloodstream (i.e., zonation), contributing to liver homeostasis. Despite the knowledge that solid cancers remotely disrupt liver homeostasis, it remains unexplored whether solid cancers affect liver zonation. Here, using spatial transcriptomics, we thoroughly investigate the abundance and zonation of hepatic genes in cancer-bearing mice. We find that breast cancers affect liver zonation in various distinct manners depending on biological pathways. Aspartate metabolism and triglyceride catabolic processes retain relatively intact zonation patterns, but the zonation of xenobiotic catabolic process genes exhibits a strong disruption. The acute phase response is induced in zonated manners. Furthermore, we demonstrate that breast cancers activate innate immune cells in particular neutrophils in distinct zonated manners, rather than in a uniform fashion within the liver. Collectively, breast cancers disorganize hepatic transcriptomes in zonated manners, thereby disrupting zonated functions of the liver.
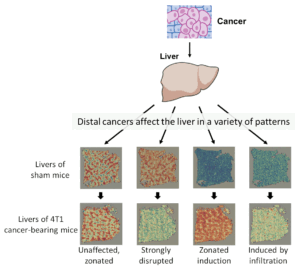
Figure: Spatial transcriptomics data revealed how a distal 4T1 cancer affects biological pathways in the liver in a variety of patterns. From left to right: 1) the zonated expression of Albumin is relatively unaffected. 2) Some pathways, such as genes involved in xenobiotic catabolism are strongly disrupted. 3) Acute phase response genes are strongly induced in hepatocytes and follow a zonated pattern. 4) A subset of immune-related genes is induced in the liver through the infiltration of neutrophils.
