| Apr 11, 2019 Comprehensive identification of cancer drug targets using CRISRP-Cas9 screens |
Fiona M. Behan 1,2、Francesco Iorio 1,2,3、Gabriele Picco 1、Emanuel Gonçalves 1、Charlotte M. Beaver 1、Giorgia Migliardi 4,5、Rita Santos 6、Yanhua Rao 7、Francesco Sassi 4、Marika Pinnelli 4,5、Rizwan Ansari 1、Sarah Harper 1、David Adam Jackson 1、Rebecca McRae 1、Rachel Pooley 1、Piers Wilkinson 1、Dieudonne van der Meer 1、David Dow 2,6、Carolyn BuserDoepner 2,7、Andrea Bertotti 4,5、Livio Trusolino 4,5、Euan A. Stronach 2,6、Julio SaezRodriguez 2,3,8,9,10、 Kosuke Yusa 1,2,11、Mathew J. Garnett 1,2
(1 Wellcome Sanger Institute, 2 Open Targets, 3 European Molecular Biology Laboratory, 4 Candiolo Cancer InstituteFPO, 5 Department of Oncology, University of Torino, 6 GlaxoSmithKline Research and Development, Stevenage, 7 GlaxoSmithKline Research and Development, Collegeville, 8 Faculty of Medicine, RWTH Aachen University, 9 Institute for Computational Biomedicine, Heidelberg University, 10 Heidelberg University Hospital, 11 Laboratory of Stem Cell Genetics, Department of Biosystems Science, Institute for Frontier Life and Medical Sciences, Kyoto University)
”Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens”
Abstract
Cancer has been the leading cause of death for the past 40 years in Japan and, due to the population aging, the number of deaths from cancer has been consistently increasing. Globally, cancer is the second leading cause of death and there was an estimate of 9.6 million deaths in 2018. The major treatments of cancer include cytotoxic chemotherapy and radiotherapy, which affect not only cancer cells but normal cells, causing serious side effects in some patients. The recent progress in understanding the molecular mechanism of cancer cell proliferation and metastasis has led to the development and clinical application of molecularly targeted drugs. This class of drugs suppress the functions of gene products that is essential for cell growth or metastasis of cancer cells and thus adverse effects of these drugs could be minimized. Although promising, the development of such drugs costs 0.2 to 1 billion US dollars per approved drug as well as requires a long R&D time of over 10 years. In addition, around 90 per cent of the drug projects fail during development. Therefore, selecting a good drug target at the beginning of the development process would be the most important part of drug discovery.
In this study, the team has conducted one of the largest CRISPR-Cas9 screens of cancer genes to date, disrupting nearly 20,000 genes in over 300 cancer models from 30 cancer types to identify critical genes for cancer cell survival and proliferation. Scientists identified several thousand key cancer genes and developed a prioritization system to narrow down the list to approximately 600 genes that showed the most promise for drug development.
One of the top-scoring targets was Werner syndrome RecQ DNA helicase (WRN). This gene product was found to be required specifically for cancer cells with microsatellite instability caused by the defective DNA mismatch repair system. Microsatellite instability occurs in many different cancer types, including 15% of colon and 28% of stomach cancers. The team found that the helicase activity of WRN is required to support cancer cell proliferation and WRN depletion led tumor regression in xenograft models. The new identification of WRN as a promising drug target offers an exciting opportunity to develop the first cancer treatments to target WRN.
|
|
|
|
Legends
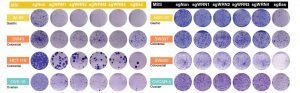
Figure 1. MSI tumor cells require WRN for their proliferation.
The WRN gene was knocked out by the CRISPR-Cas9 technology in microsatellite instable (MSI) or stable (MSS) cell lines and the cells were analyzed by clonogenic survival assay. MSI cell lines showed a marked proliferation defect (sgWRN1-4). sgNon, non-targeting negative control sgRNA; sgESS, positive control sgRNA targeting a gene essential for cell survival.
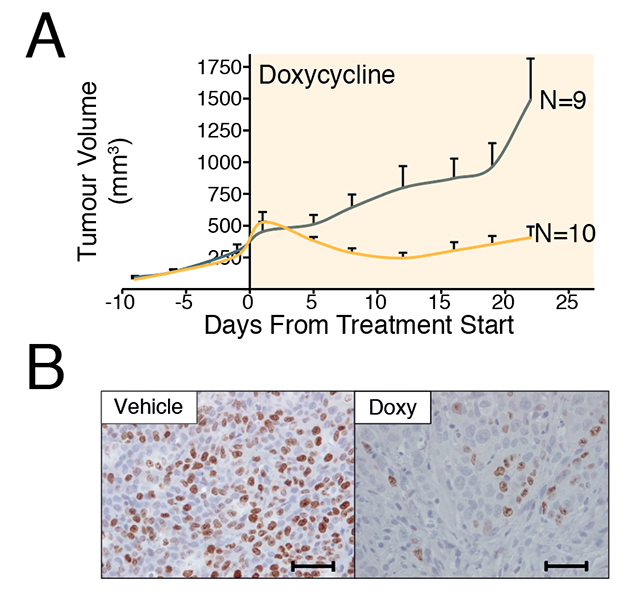
Figure 2. in vivo tumor growth assay.
A. HCT-116 cells that express sgRNA targeting WRN in a doxycycline-dependent manner were transplanted subcutaneously in immunodeficient mice. When the tumor size reached a set size, sgWRN was induced by administrating the mice with doxycycline to knockout the WRN gene. Upon induction, significant tumor regression was observed.
B. In WRN-depleted tumors, the number of Ki67-positive cells were significantly reduced.