| Nov 02, 2022 Development of an in vivo cleavable donor plasmid for targeted transgene integration by CRISPR-Cas9 and CRISPR-Cas12a |
Riki Ishibashi1,2*, Ritsuko Maki1, Satsuki Kitano3, Hitoshi Miyachi3 and Fumiko Toyoshima1,2*
1Department of Biosystems Science, Institute for Life and Medical Sciences, Kyoto University
2Department of Mammalian Regulatory Networks, Graduate School of Biostudies, Kyoto University
3Reproductive Engineering Team, Institute for Life and Medical Sciences, Kyoto University
Development of an in vivo cleavable donor plasmid for targeted transgene integration by CRISPR-Cas9 and CRISPR-Cas12a
Scientific Reports (2022) https://doi.org/10.1038/s41598-022-22639-6
Abstract
The CRISPR-Cas system is widely used for genome editing of cultured cells and organisms. The discovery of a new single RNA-guided endonuclease, CRISPR-Cas12a, in addition to the conventional CRISPR-Cas9 has broadened the number of editable target sites on the genome. Here, we developed an in vivo cleavable donor plasmid for precise targeted knock-in of external DNA by both Cas9 and Cas12a. This plasmid, named pCriMGET_9-12a (plasmid of synthetic CRISPR-coded RNA target sequence-equipped donor plasmid-mediated gene targeting via Cas9 and Cas12a), comprises the protospacer-adjacent motif sequences of Cas9 and Cas12a at the side of an off-target free synthetic CRISPR-coded RNA target sequence and a multiple cloning site for donor cassette insertion. pCriMGET_9-12a generates a linearized donor cassette in vivo by both CRISPR-Cas9 and CRISPR-Cas12a, which resulted in increased knock-in efficiency in culture cells. This method also achieved > 25% targeted knock-in of long external DNA (> 4 kb) in mice by both CRISPR-Cas9 and CRISPR-Cas12a. The pCriMGET_9-12a system expands the genomic target space for transgene knock-in and provides a versatile, low-cost, and high-performance CRISPR genome editing tool.
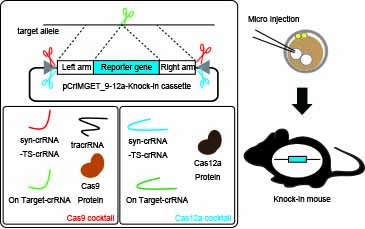
(Figure 1) Scheme of the knock-in mice generation via pCriMGET_9-12a system
